Introduction: Allogeneic hematopoietic cell transplantation (HCT) is curative for myelofibrosis (MF). However, prognosis post HCT is variable. Identifying subsets of patients who can greatly benefit from HCT is important for clinical decision-making and patient counseling. The Dynamic International Prognostic Scoring System (DIPSS) score includes cytogenetic abnormalities provides important prognostic information. Here, we used high-resolution single nucleotide polymorphism (SNP) arrays to characterize chromosomal aberrations in pre-HCT blood samples from patients with MF and evaluated their added value in patient risk stratification for post HCT survival.
Methods: We used the Illumina Global Screening Array to detect somatic copy number alterations (SCNAs) sized ≥2Mb (by calculating the log2 R ratio and B allele frequency and leveraging haplotype information) in pre-HCT blood samples (collected 2-4 weeks prior to HCT conditioning regimen administration) from 923 MF patients who underwent HCT between 2000-2016. Clinical data and blood samples were obtained from the Center for International Blood and Marrow Transplant Research. We used Kaplan-Meier survival analysis and multivariable Cox regression to evaluate the prognostic significance of observed SCNAs on survival after HCT.
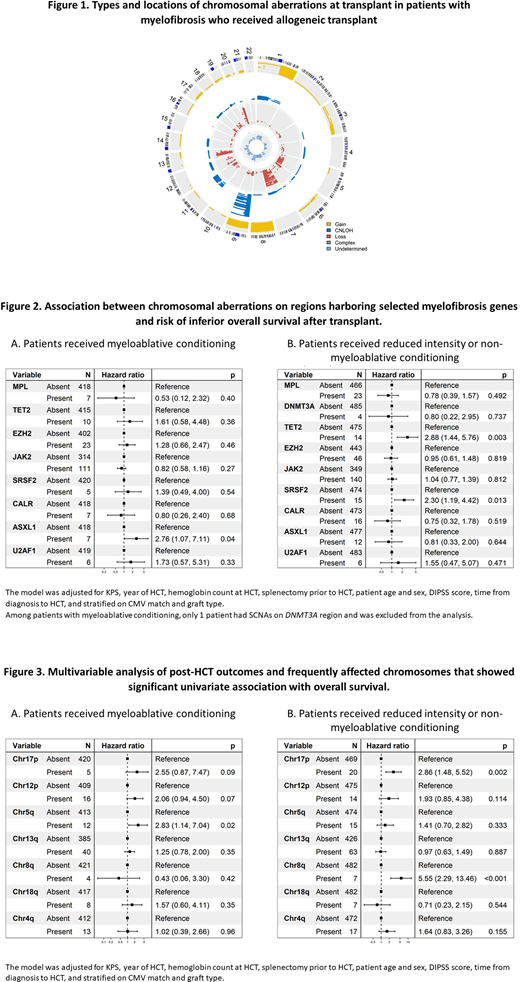
Results: Median age at HCT was 58 years (range=0.7-76). About 58% were males (N=536), 85% received unrelated donor HCT (N=787), 90% received peripheral blood stem cell grafts (N=826), 47% (N=429) received myeloablative conditioning (MAC), 34% (N=312) received HCT within 1 year from diagnosis, and 537 patients (58%) had known DIPSS score at HCT (47% of them were intermediate II or high). A high frequency of SCNAs were noted in MF (N=623; 67.5%), most commonly on chromosome (chr) 9p (N=224, 24%), chr13q (N=104, 11%), and chr20q (N=102, 11%) (Figure 1). Aberrations noted on chromosomal regions harboring the known MF genes (MPL, DNMT3A, TET2, EZH2, JAK2, SRSF2, CALR, ASXL1, U2AF1) accounted for 28% of all detected (40% of the patients), including JAK2 (9p24.1, N=253, 27%), MPL (1p34.2, N=30, 3.3%) and CALR (19p13.13, N=23, 2.5%). Multivariable models including all MF genes (adjusted for DIPSS score, KPS, year of HCT, hemoglobin count, splenectomy, age, sex, time from diagnosis to HCT, and stratified on CMV match, graft type and conditioning regimen) showed an association between SCNAs on TET2 or SRSF2 regions and poor survival (HR for TET2 =2.40, 95% CI=1.33-4.32, p=0.004; and HR for SRSF2= 2.22, 95% CI=1.26-3.89, p=0.006). TET2 region aberrations were associated with an increased risk of transplant-related mortality (TRM) (HR=2.97, 95% CI=1.53-5.78, p=0.001), worse disease-free survival (DFS; HR=2.22, 95% CI=1.33-3.69, p=0.002), but not the risk of relapse (p=0.12). SRSF2 region aberrations were associated with DFS (HR=1.94, 95% CI=1.11-3.37, p=0.02); no statistically significant association was noted with the risk of relapse (p=0.07) or TRM (p=0.44). These associations were predominant in patients receiving reduced intensity (RIC) or non-myeloablative regimens (Figure 2).
Further univariate analyses focusing on all frequently affected chromosomes (affecting ≥10 patients) showed poor OS with SCNAs on chr17p (log-rank p<0.0001), 12p (p<0.0001), 5q (p<0.0001), 13q (<0.001), 8q (p=0.004), 18q (p=0.02) and 4q (p=0.03). In multivariable models stratified on conditioning regimens, chr17p aberrations showed a significant association with excess risk of relapse independent of regimen intensity (HR=5.31, p=0.01 in MAC, and HR=2.70, p=0.02 in RIC). The HR for OS was 2.55, p=0.09, and 2.86, p=0.002, respectively. Other chromosomal aberrations that showed significant associations with inferior OS in patients receiving RIC were SCNAs on chr8q (HR=5.55, p<0.001) (Figure 3), with excess risk of TRM (HR=5.09, p=0.002) but not relapse (p=0.34). In patients that received MAC, elevated risk of mortality was noted with chr5q SCNAs (HR=2.83, p=0.02) with a suggestive association with relapse (HR=2.72, p=0.08) but not with TRM (p=0.59).
Conclusion: This study highlights the prognostic ability of pre-HCT detected SCNAs in patients with myelofibrosis, independent on DIPSS score. Relapse risk in patients with aberrations affecting TP53 region was not eliminated by MAC. Follow-up studies to confirm the poor prognosis of those chromosomal changes are warranted.
Lee:Incyte: Consultancy, Research Funding; Syndax: Research Funding; Kadmon: Research Funding; AstraZeneca: Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Research Funding; Takeda: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal